
Electronegativity
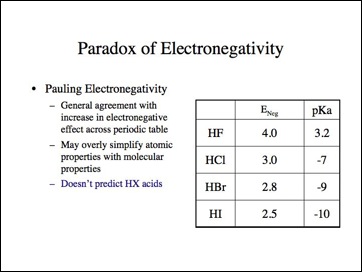
Organic chemists know that tertiary carbocations are more stable. Similarly, every organic chemist knows iodides are better leaving groups and more reactive than bromides and in turn chlorides, and much more than fluorides. The notion that fluoride has more anionic character in a reaction in which the slow step is formation of an anion is counterintuitive. They also know that tertiary butyllithium is extremely reactive and methyllithium is more stable. Simply, if we were making electronegativity arguments, iodine would be more electronegative than fluoride and hydrogen is more electronegative than carbon. The electronegativity should follow the acidity of HI (pka ~-10), a stronger acid than HF (pKa ~3), and hydrogen (pKa ~35, NaH) a stronger acid than methane (pKa ~50).
When I began teaching and to teach electronegativity, I was uncomfortable with how it fit in with real chemistry, with reactions, with reactivity and with reaction products. I looked up Pauling's 1932 paper on electronegativity. Pauling knew that some compounds were more stable than expected. Fluorine had the greatest difference. He thought the increase in stability of fluoride and other atoms was due to Coulombic attraction of the ions. In 1932, that idea was ingenious. The electronegativity values matched what chemists knew, F>O>N>C. The electronegativity values must be correct, right?
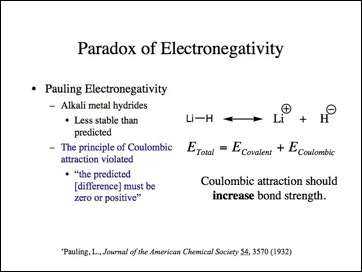
When I read the paper, I knew some compounds did not match the profile asserted by Pauling. Pauling stated that Coulombic attraction can only increase bond strength and at its lowest, no effect. Didn't the metal hydrides disprove the electronegativity principle? This had been considered the paradox of electronegativity. To resolve this problem, Pauling squared the terms in order to change the sign of the effect on bond strength. The decrease in bond strength was treated as though it increased it. I am not the first to note this.
What I am not aware of is anyone disagreeing with Pauling's conclusion with his rational for increased bond strength and to suggest a reason why fluoride might have strong bonds and metal hydrides might be weaker. Below is a synopsis of a presentation from the Philadelphia ACS meeting in 2004.
Since then, I have removed some aspects of the argument to make it less controversial (I hope). The basic ideas are present in Chapter 1 of "The Language of Organic Chemistry" but with no mention of electronegativity.
I was also prompted to write this in response to some student discussion about electronegativity.
From my ACS presentation, below are relevant slides.
What were the signs of the times?

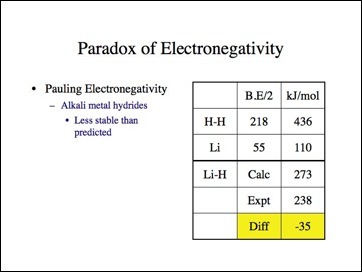
This is the data Pauling used.
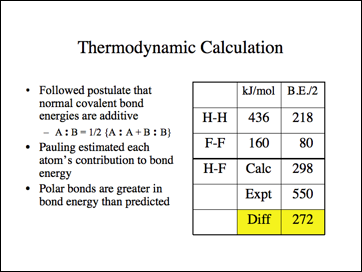
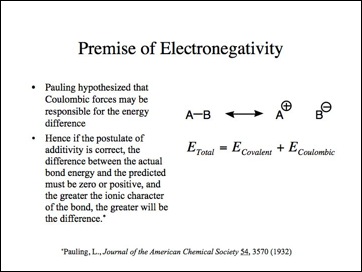
This is Pauling's hypothesis for this energy difference.
A comparison of electronegativity values and pKa values.
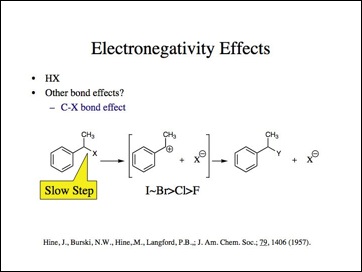
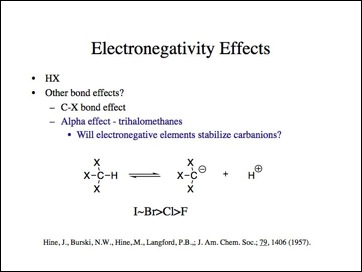
Maybe the H-X bond is anomalous. Lets look at the carbon-halogen bond.
Lets look at the effect if we move the electronegative element more distant to the bond that changes. Now, it should not be a function of the actual bond and its length, but an inductive effect.
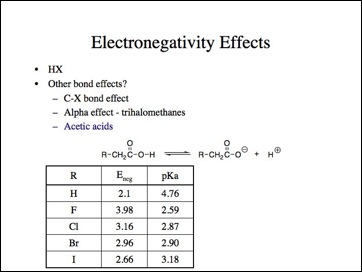
Lets look at moving the electronegative atom further away, such as the acetic acids. This is an often cited example and see, fluorine is more acidic because it is more electronegative.
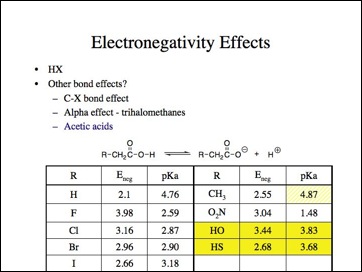
Oh, oh, nitroacetic is a stronger acid than fluoroacetic acid and hydroxyacetic and thiolacetic acid are out of order and less than all of the haloacetic acids. Propionic acid is also less acidic. Looks like another anomaly.
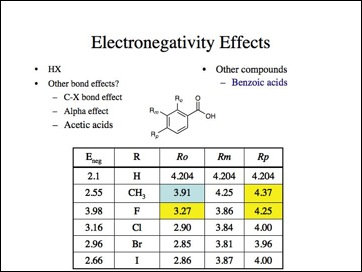
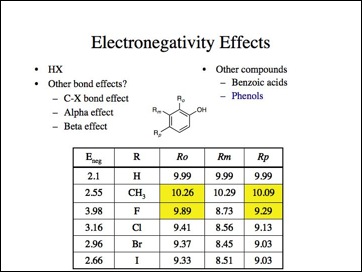
Lets look at the effect on other bonds, obviously I am selectively picking my data, right?
Oh no, not more bad data. Note, methyl is more electronwithdrawing here. (Probably because it is ortho and interferes with the interaction of the carboxylate group and the aromatic ring.) Fluorine is the least electronegative atom (again).
More bad data. Anyone care to look an anilines? I skipped them, but the effect is the same, fluorine is the least electronegative and carbon less than hydrogen.
Lets look at lithium hydride. Remember the difference must be positive?
It must be positive because lithium (positive) and hydride (negative) must be attractive in an ionic bond according to the electronegativity theory.
Is it possible that Pauling was wrong?
Is this a good model for atomic structure? Aren't there multiple charges present? Is the separation of ionic charges a good model to predict bond strength?
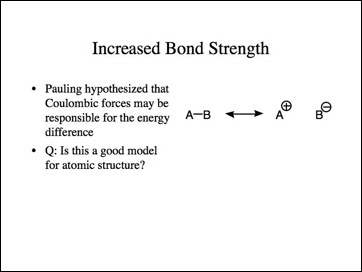
Let me suggest another simple model for bond strength consistent with Coulomb's Law. If nucleus q1 pulled the bonding electrons q2 closer to it, those electrons would be held more strongly. Conversely, if the electrons moved away from q3, they would be held less tightly. The effect is that q1 would have a negative charge and q3 a positive. This is another model for an ionic molecule.
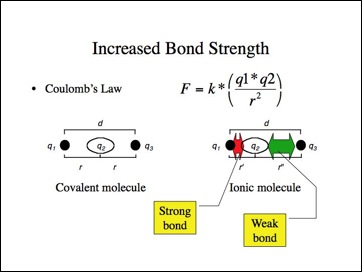
If the ionic bond model were applied to the halides, they should have shorter (stronger) bonds than predicted while the metal hydrides have longer (weaker) bonds than predicted? While it is difficult to find consistent data for different bond lengths, I must leave you to answer that question. The metal hydride bonds lengths appear to be longer, but it depends upon how the data was gathered and whether other bond lengths are determined in the same manner.
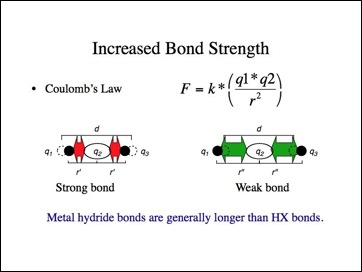
Okay, that may work for the halogens, but carbon is more electronegative than hydrogen, right?
Lets go back to the examples. We know methyl carbocation is less stable than a tertiary and methyl anion is more stable then tertiary.
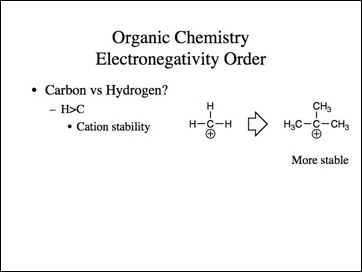
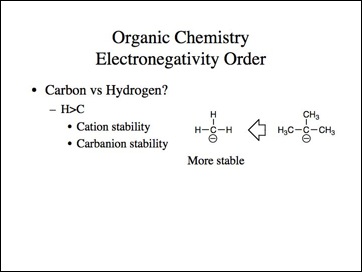
Uncle?
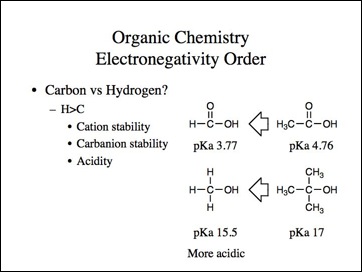
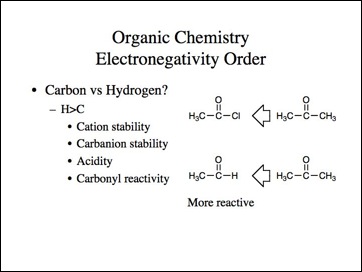
This is just the first part of my thoughts on atoms and bonding. I confess many of the other ideas are equally avant-garde. It is my objective to make those ideas the subject matter of another book.
PW